CoStorSol™
A Cold Storage Solution
Sterile Medical Device Using Aseptic Technique (Aseptic Fill).
Directions for Preparation and Use
NOT FOR DIRECT INJECTION OR INTRAVENOUS INFUSION.
Description
CoStorSol® (University of Wisconsin) Cold Storage Solutions’ composition is:
- Pentafraction: 50 g/L
- Lactobionic Acid (as Lactone): 35.83 g/L
- Potassium Phosphate monobasic: 3.4 g/L
- Magnesium Sulfate heptahydrate: 1.23 g/L
- Raffinose pentahydrate: 17.83 g/L
- Adenosine: 1.34 g/L
- Allopurinol: 0.136 g/L
- Total Glutathione: 0.922 g/L
- Potassium Hydroxide: 5.61 g/L
- Sodium Hydroxide/Hydrochloric Acid: Adjust to pH 7.4
- Water for Injection: q.s.
CoStorSol® is a clear to light yellow, sterile, non-pyrogenic solution for hypothermic flushing and storage of organs. The solution has an approximate calculated osmolarity of 320 mOsm, a sodium concentration of 29 mEq/L, a potassium concentration of 125 mEq/L, and a pH of approximately 7.4 at 20°C.
How Supplied - Supply Conditions
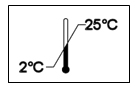
1000 mL CoStorSol® (University of Wisconsin) Cold Storage Solution in one liter bags, shelf carton of 10. Store CoStorSol® indoors at temperatures controlled between 2 ° and 25°C (36° and 77°F). Avoid excessive heat. Do not freeze the solution, and
do not use if frozen. Do not use if discolored or if obvious particulate matter, precipitates, or contamination are evident in the solution.
Actions
CoStorSol® must be cooled to 2° to 6°C (36° to 43°F) prior to use. The cold solution is used to flush the isolated organ immediately before removal from the donor and/or immediately after removal from the donor. The solution is then left in the organ vasculature during hypothermic storage and transportation. CoStorSol® is to be used for cold storage of the organ and is not acceptable for continuous machine perfusion. Administration of CoStorSol®, at the recommended temperature, will effectively cool the organ and lower its metabolic requirements.
Intended Use
CoStorSol® is intended for the flushing and cold storage of kidney, liver and pancreas organs at the time of organ removal from the donor in preparation for storage, transportation and eventual transplantation into a recipient.
Contraindications
There are no known contraindications when used as directed.
Warning
NOT INTENDED FOR DIRECT INJECTION OR INTRAVENOUS INFUSION.
Precautions
The donor organ must be flushed free of the CoStorSol® prior to the reperfusion. The organ must be flushed with physiological solution to prevent occurrence (in the recipient) of potentially serious cardiovascular complications such as hyperkalemic cardiac arrest or bradyarrhythmia. This is necessary because of the high concentration of potassium in the solution. These precautions must be taken during donor organ retrieval to avoid cardiac arrest.
CoStorSol® includes components (allopurinol and pentafraction) which individually have caused hypersensitivity reaction in patients. Additionally, the additives recommended for use with CoStorSol® (penicillin, insulin, and dexamethasone) have individually been associated with hypersensitivity reactions in patients. Physicians should consult individual drug labeling and be alert to treat possible reactions.
Adverse Reactions
Cardiovascular complications such as bradyarrhythmia have been reported in cases where the organ has been reflushed with fresh solution within a short period (1 to 3 hours) prior to release of vascular anastomosis clamps in the recipient, or when inadequate flush-out of the solution has occurred.
A few anecdotal reports when this solution was used in liver graft preservation described clinical problems including hepatic functional changes, poor outcomes including death, and biopsies showing ischemic damage in the liver with or without signs of mild rejection.
Preparation and Administration With Liver, Kidney and Pancreas
Cool the solution to 2° to 6°C (36° to 43°F). Remove overwrap prior to use. Check each bag for leaks by squeezing the container firmly. If a leak is found, discard solution container. With the overwrap removed, perform a visual inspection of the solution for particulate matter. Do not use the solution if obvious particulate matter, precipitates, or contamination are evident in the solution.
Immediately prior to use, aseptically add the following additives:
- Penicillin G, 200,000 units
- Regular Insulin, 40 units
- Dexamethasone, 16 mg
Glutathione, one of the components of CoStorSol®, oxidizes during storage. If desired, an additional 0.922 g/L (3 mmole/L) of glutathione may be added if Transplant Center policy or Surgical Personnel requirements call for its use. (Boudjema et al, Transpl. Proc. 23[5]1991; Merion et al, Transpl. Proc. 23[4]1991).
Remove protective cap from the bag outlet port designated delivery set port. Insert the spike from the administration set into the bag port with a twisting motion. Open clamp on administration set. Hold the administration set vertically above the solution bag, then squeeze the solution bag into the administration set. Close the clamp.
Prior to connection to the organ, the solution container should be suspended from a sufficient height to allow for a steady stream of solution and to produce flow rates of at least 30 mL/min during flushing. Open the clamp to begin flushing. Flushing should be continued until the organ is uniformly pale and the effluent is relatively clear.
Suggested Minimum Volumes
In situ aortic flush:
Adults, 2-4 L
Infants, 50 mL/kg
Ex vivo infusion: liver (via portal vein and biliary tree)
Adults, 1200 mL
Infants, 50 mL/kg
Pancreas or Kidney:
Adults, 300-500 mL
Infants, 150-250 mL
Additional solution should be dispensed into the container holding the organ. Seal the container aseptically. The organ storage container should be maintained within a well-insulated transport container. Ice should be used to surround the organ storage container, but should not be used within the container, where the ice could come into direct contact with the organ. Donor organs must be flushed free of CoStorSol® prior to anastomosis (Refer to PRECAUTIONS Section.). In order to minimize residues of the solution in the liver, just prior to anastomosis, flush one liter of Lactated Ringer’s through the hepatic portal vein.
Ischemia Times
The recommended following times for each organ are:
Cold Ischemia Times
Liver: not longer than 17 hours
Kidney: not longer than 23 hours
Pancreas: not longer than 21 hours
Warm Ischemia Times
Liver: not longer than 2.5 hours
Kidney: not longer than 2.5 hours
Pancreas: not longer than 2.5 hours
Caution
Federal (USA) law restricts this device to sale by or on the order of a physician.