MaPerSol™
Organ Preservation Solution
Sterile Medical Device Using Aseptic Technique (Aseptic Fill).
Indications for Use
MaPerSol® Organ Preservation Solution is intended for the in-vitro flushing and continuous hypothermic machine perfusion preservation of explanted kidneys.
Suggested Volume
Preservation Solutions, Inc. recommends a volume of 1000 mL of MaPerSol® perfusate (one bag) for two (2) human kidneys.
Device Description
MaPerSol® Organ Preservation Solution is a clear to straw-colored solution for the in-vitro flushing and temporary continuous perfusion preservation of explanted kidneys. This solution is consistent with an extracellular solution, based on its sodium/potassium ratio. This solution has a calculated potassium concentration of 25 mEq/L, a sodium concentration of 100 mEq/L, an osmolarity of 300 mOsM, and a pH of 7.4 ±1.0 at room temperature.
Storage Conditions
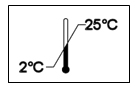
MaPerSol® solution should be stored between 2° - 25°C (35.6° - 77°F). While 5°C is the ideal temperature for actual perfusion of MaPerSol®, a range of 4 ° - 8°C is acceptable. Do not freeze or expose to excessive heat.
Preparation and Administration
Inspect perfusate to ensure there is no particulate matter, precipitates or contamination in the perfusate. If the perfusate is clear and no particulate is observed, the perfusate is safe to use.
Note: If the perfusate contains any particulate, contact Preservation Solutions, Inc to
make arrangements to return.
Pre-cool the kidney by vascular flush-out using MaPerSol® or other cooled solutions (2°- 8°C) (ViaSpan™, Ringers, or saline). The kidney can then be placed into a perfusion apparatus that is capable of maintaining temperature within the range of 2 ° - 8 °C. The kidney should be perfused following the manufacturers or perfusionists protocol. MaPerSol® is suitable for a mean perfusion time of 29 hours ±8 hours1. MaPerSol® should be flushed from the donor organ at the time of implantation.
For further information regarding clinical experience with organ preservation solutions, please contact the company for a bibliography of organ preservation articles.
Additives
Possible additives, recommended by the University of Wisconsin include: Penicillin (150,000 units), Regular Insulin (40 units) and Dexamethasone (8 mg).
Precautions
MaPerSol® is made with Hydroxyethyl Starch, which has been the cause of hypersensitivity reactions in patients. Also, if applicable penicillin, insulin and dexamethasone have caused hypersensitivity reactions in patients. Physicians should be prepared to respond to possible reactions.
Warning: This product is NOT intended for direct injection or I.V. use.
Solution Composition
Constituent |
Amount/1000 ml |
Concentration (mM) |
Adenine (free base) |
0.68 g |
5 |
Calcium Chloride (dihydrate) |
0.068 g |
0.5 |
Dextrose (+) |
1.80 g |
10 |
Glutathione (reduced) |
0.92 g |
3 |
HEPES (free acid) |
2.38 g |
10 |
Hydroxyethyl Starch |
50.0 g |
N/A |
Magnesium Gluconate (anhydrous) |
1.13 g |
5 |
Mannitol |
5.4 g |
30 |
Potassium Phosphate (monobasic) |
3.4 g |
25 |
Ribose, D(-) |
0.75 g |
5 |
Sodium Gluconate |
17.45 g |
80 |
Sodium Hydroxide |
0.70 g |
N/A |
Sterile Water for Injection |
To 1000 mL Volume |
N/A |
Adverse Reactions
When MaPerSol® solution is used as described, no adverse reactions attributed to the solution have been observed.
Caution
Federal and certain international laws restrict the sale of MaPerSol® to the order of a physician or a licensed practitioner.
1WH Barber et. al.: Comparison of Simple Hypothermic Storage, Pulsatile Perfusion with Belzer's Gluconate-Albumin Solution, and Pulsatile Perfusion with UW Solution for Renal Allograft Preservation. Transplantation Proceedings, Vol. 23, No.5 (October), 1991; pp 2394-2395.